Water: H2O
Carbon Dioxide: CO2
Methane: CH4
2. Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat. Explore the ingredients of things like cosmetics and foods.
1. Water: H2O
3. (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol (Sucrose): C12H22O11
4. 7,8-dimethyl-10-[(2R,3R,4S)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4(3H,10H)-dione (Riboflavin): C17H20N4O6
4. 7,8-dimethyl-10-[(2R,3R,4S)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4(3H,10H)-dione (Riboflavin): C17H20N4O6
5. Nicotinic acid (Niacin): C6NH5O2
6. 3-[(2,4-dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid (Pantothenic Acid): C9H17NO5
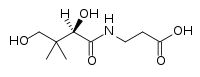
6. 3-[(2,4-dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid (Pantothenic Acid): C9H17NO5

7. [(4-formyl-5-hydroxy-6-methylpyridin-3-yl)methoxy]phosphonic acid (Pyridoxal Phosphate): C8H10NO6P


8. (2S)-2-[(4-{[(2-amino-4-hydroxypteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid (Folic Acid): C19H19N7O6


10. Calcium: Ca
13. Phosphorus: P


16. 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione (Caffeine): C8H10N4O2
18. trihydroxidooxidophosphorus (phosphoric acid): H3PO4


20. Sodium nitrate:NaNO3
3. Look over your molecules and the bonding characteristics, how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon: 4
Hydrogen: 1
Oxygen: 2
4. What does IUPAC stand for?
International Union of Pure and Applied Chemistry
5. As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be?
Companies make their products using only natural products that form a bond but do not have any harsh chemicals in it.















No comments:
Post a Comment